Reduce the Harm
of Tobacco Products
For decades we’ve been leaders in the tobacco industry, but the industry is evolving - and so are we. We’re Moving Beyond Smoking®.
There are 55 million U.S. adult nicotine consumers 21+ today, and more than ever they’re seeking smoke-free options that fit their lifestyles. We’re here to meet that demand, making a meaningful impact in harm reduction while delivering products people love. This is a transformative business opportunity. We want to be the leader in nicotine enjoyment for adults.
We believe adult nicotine consumers deserve options – options that satisfy and delight them, that keep pace with their evolving expectations, and that can potentially reduce risk. And they deserve accurate information so they can make informed choices.
Our 2025 Goals
- Accelerate investments in innovation, science and regulatory support for new smoke-free product platforms.
- Establish harm reduction, not prohibition, as the proper framework for tobacco regulation in the United States.
- Provide access to expert quitting information for those who have decided to quit.
Our Progress
We’ll deliver compelling, innovative smoke-free products and brands across e-vapor, oral nicotine pouches and heated tobacco. In each of these categories we’ll fight hard to earn consumer love and loyalty, grow our share and win. We’ll help transition adult smokers to a smoke-free future and we’ll vigorously compete for existing smoke-free adult nicotine consumers 21+. With urgency, determination, and a commitment to responsibility, we’ll shape the future of nicotine enjoyment.
This includes advocating for a harm reduction future in which the industry is operating within science-based regulation, underage tobacco use continues to decline, and adult smokers who can’t or won’t quit are moving to smoke-free alternatives that are authorized by the U.S. Food & Drug Administration (FDA). Importantly, we'll work within the framework that government, public health and regulatory bodies have established to communicate about reduced harm choices. And for any tobacco consumer who wants to quit, we offer access to a breadth of information from experts on how to do so successfully. Learn More
We know that millions of adult smokers 21+ are interested in completely switching from cigarettes to a smoke-free tobacco product. And we need to be sure that all adult smokers, regardless of background, demographics or financial means, have equitable opportunities to reduce the harm of smoking.
Did you Know?
Significant majorities of U.S. adults 21+ surveyed believe a wide variety of FDA regulated smoke-free product choices are needed to help adults who smoke move beyond smoking.*
95% of smoke-free product only consumers
88% of health care providers
83% of cigarette only consumers
81% of general population adults
FDA's Regulatory Authority over Tobacco Products
The FDA regulates all aspects of the manufacturing, distribution and marketing of tobacco products. With the FDA's expansive authority to define the tobacco marketplace, the Agency is in the leading position to create a mature, regulated tobacco marketplace that successfully reduces the harm caused by cigarettes by:
- timely scientific review and authorization of potential reduced risk-tobacco products;
- actively enforcing existing regulations and taking action to eliminate illicit markets;
- monitoring manufacturers' post-market surveillance data and quickly identifying and remediating emerging issues (e.g., uptick in underage use of a newly authorized product);
- helping to correct nicotine misperceptions and communicate the relative risks of different products to encourage adult smoker transition to smoke-free products; and
- investing in proven tactics to prevent and reduce underage tobacco use.
However, for tobacco harm reduction to succeed, we need a fully functioning regulatory system. 2025 marks 16 years since Congress granted the FDA authority to regulate tobacco products. We supported FDA regulation because we believed science- and evidence-based federal regulation would help address societal concerns about tobacco products, promote public health and benefit adult tobacco consumers. While we still believe federal regulation is critical for harm reduction to succeed, we have a long way to go to realize its promise.
Adult smokers seeking potentially reduced harm products have a right to accurate, science-based information about those products, yet consumers are misinformed about the role of nicotine.
The FDA has authorized very few innovative smoke-free products, woefully insufficient to meet the growing adult tobacco consumer demand. And the marketplace is being overrun by illicit disposable e-vapor products that are flouting FDA regulation and contributing to underage use.
To make harm reduction a reality, we are engaging extensively with the FDA and other key stakeholders to advocate for policies and actions that will turn the tide on illicit vapor activity and accelerate a positive impact on public health. Through our efforts and those of others, we hope to see faster progress from the FDA in authorizing smoke-free products, informing adult smokers about the benefits of switching to smoke-free alternatives and enforcing the law by ridding the marketplace of illicit e-vapor products. These changes won't happen overnight, but we're committed to doing our part by engaging and leading responsibly.
Did you Know?
Most U.S. adults 21+ surveyed expect the FDA to urgently replace the illicit market with a legal and regulated one that responsibly meets adult nicotine consumer 21+ demand.*
92% of smoke-free product only consumers
85% of health care providers
79% of cigarette only consumers
77% of general population adults
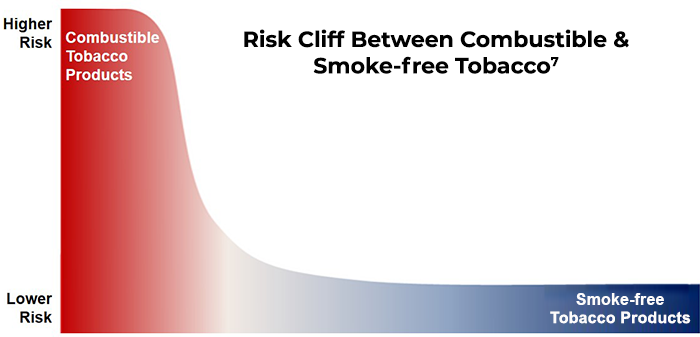
Nicotine Misperceptions & The "Risk Cliff"
Persistent misperceptions regarding the role of nicotine and the relative risks of different tobacco products prove to be a significant obstacle to adult smokers' switching to potentially reduced-harm products.
As the FDA states on its website: "Many people who use tobacco products have misperceptions about nicotine and the risks of various tobacco products.
Despite science that shows that e-cigarettes generally have lower levels of harmful ingredients than cigarettes, many adults believe that e-cigarettes are just as harmful or more harmful than cigarettes.
Research has found that some adults who perceive e-cigarettes to be as or more harmful than cigarettes are less likely to switch from cigarette smoking to exclusive e-cigarette use."4
Did you Know?
Majority of U.S. adults 21+ surveyed incorrectly believe that nicotine is a carcinogen, a cancer-causing chemical.*
87% of general population adults
83% of cigarette only consumers
75% of smoke-free product only consumers
70% of health care providers
And U.S. adults 21+ surveyed feel it’s particularly urgent for the FDA to educate stakeholders with accurate and truthful information about the role of nicotine, the lower relative risk of smoke-free nicotine products, and the benefits of switching completely to them.*
88% of health care providers
81% of smoke-free product only consumers
80% of general population adults
73% of cigarette only consumers
The FDA and other public health authorities agree that there is a broad "continuum of risk" among tobacco products, with cigarettes at the highest end of that spectrum and complete cessation at the lowest end.5 While there may be differences in risk profiles between specific smoke-free products, it will take years of epidemiology to quantify those differences. The body of evidence does, however, indicate a profound risk differential between combustible and smoke-free product categories.
Data suggest that a two-dimensional "Risk Cliff" model is a useful description of the relative risk between combustible products (such as cigarettes and cigars) and smoke-free products (such as moist smokeless tobacco, heated tobacco, e-vapor and oral nicotine pouches).
When the FDA announced its Comprehensive Plan in 2017, then-FDA Commissioner Gottlieb stated FDA policy should be used as a vehicle to "move addicted smokers down that continuum of risk to these less harmful [innovative] products."6 The then-Commissioner also shared his concerns regarding the widespread misperceptions around nicotine among the American public.
The FDA, the public health community and tobacco manufacturers all have a role to play in addressing misinformation that hinders progress on harm reduction. We believe it is our responsibility to help create the conditions for harm reduction to succeed – through education, awareness and advocacy – as we build a strong portfolio of smoke-free products that satisfy adult smokers’ evolving interests and preferences.
You May Also Like
* Altria Client Services Tobacco Harm Reduction Engagement Survey – Findings are drawn from a nationwide survey conducted online among adults age 21+ including n=1,000 General Population (margin of error “m.o.e.” +/- 2.9%), n=438 Cigarette Only Consumers (m.o.e. +/- 4.6%), n=405 Smoke-Free Only Consumers (m.o.e. +/- 4.8%), and n=402 Health Care Providers (m.o.e. +/- 4.8%), from May 27 – June 22, 2025. The interview length averaged 17 minutes and the margins of error (m.o.e) for these samples are calculated at the 95% confidence level. The primary objective of this survey was to measure the attitudes and opinions of societal stakeholders nationwide on the public health strategy of tobacco harm reduction and the regulatory reforms needed to advance it.
1 Altria Client Services Consumer Market Insights Data
2 O'Brien, E. K., Nguyen, A. B., Persoskie, A., & Hoffman, A. C. (2016). U.S. adults' addiction and harm beliefs about nicotine and low nicotine cigarettes. Preventive Medicine, 96, 94-100. doi:10.1016/j.ypmed.2016.12.048
3
5